Theme: Novelties in Drug Formulation & Manifesting advancements in Analytical Techniques
Drug Formulation 2019
We are welcoming the participants from across the globe to attend 11th Annual Congress on Drug Formulation & Analytical Techniques during December 09-10, 2019 in Dubai, UAE Who is related to Drug industries, pharmaceutical companies. Drug Formulation 2019 is a specially designed cluster Pharmaceutical and Medicinal Chemistry Conference.
The main theme of our conference is "Novelties in Drug Formulation & Manifesting advancements in Analytical Techniques" this unique international conference will opportunity to reach the largest assemblage of participants from the Pharmaceutical community to gather and share their insights and convey recent developments in the field of generic drug research and current challenges and possibilities in modeling a new drug and breakthroughs in drug development, Generic drug safety, Novel trends and advanced strategies involving Formulation research. This is a global platform where ideas and discussion is driven by the participants and interaction with peers and others leads to fruitful outcomes.
Drug Formulation 2019 is an event for two days offering the Exhibition or workshop’s, at conference to showcase their new Research work and emerging technologies. Including that conference have wider sessions i.e., Keynote presentation, Oral, YRF (student presentation), poster, e-poster presentations. The Eminent Speakers and delegates are attending the conference from all over the globe, to share their valuable presentation’s on their most recent research work and the advanced techniques, developments, and the newest updates related to Drug Formulation are the prominent features of the conference.
Why to Attend???
Meet your target audience with members from around the world focused on learning about Pharmaceutical Sciences and Medicinal Chemistry, this is your single best opportunity to reach the largest assemblage of participants from the Pharma and medicinal community, Find out how innovators are closing the gap between product development and adoption, Better identify cyber threats and reduce information security risks, Debate the potential to greater cross-industry and cross-sector collaboration in the race for faster, cheaper, better cures. Conduct demonstrations, distribute information, meet with current and potential Scientists, make a splash with a new research, and receive name recognition at this 2-day event Pharmaceutical Science & Medicinal Chemistry. World-renowned speakers, the most recent techniques, tactics, and the newest updates in Pharmaceutical Sciences fields are hallmarks of this conference.
A Unique Opportunity for Advertisers and Sponsors at this International event:
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneur
- Government healthcare departments, HTA’s and drug regulators
- Digital health companies
Genomics and personalised medicine experts
Track 1: Pharmaceutical Formulations
Pharmaceutical Formulation in pharmaceutics is the method in which various chemical substances including the energetic drug are joined to produce a final medicinal combining the drug into a tablet or a pill. Formulations verify that the drug is compatible with those other substances in the product. formulation research involve developing a preparation of the drug which is both stable and acceptable to the patient. Having tablets orally contains pharmaceutical drug formulations, the different physical, chemical, and mechanical properties of a drug are considered so as to know what the other compound should be used within the practises. The different factors like polymorphism, particle size, pH, and solubility are all considered while formulating the drug, additionally thinking about the appearance of the tablet.
Track 2: Formulation R&D
The drug subjected to drug development undergoes number of trials and are screened at different stages to produce a final potent drug intended for the treatment of various diseases. During this process various properties are checked to see whether the drug is non-toxic to living system and is therapeutic or not. Using suitable excipients and technological preparations the active substances are formulated into final dosage form. The last product is the Actual composition of preparation, manufacturing specification. Drug formulation can have a substantial impact on almost every quality characteristic of an API including potency, bioavailability, solubility, dosage, route of administration and stability. In immune offers parenteral drug formulation R&D services for primary stage and preclinical candidates with a special prominence in nanoparticle based drug delivery technology.
Track 3: Clinical Research Vs Clinical Trails
Bioavailability is a subcategory of pharmacological absorption. Bioavailability is generally assessed by finding the area under the plasma concentration–time curve. The factors affecting bioavailability are Pharmaceutics factors, physicochemical properties of drug, Dosage form characteristics & Pharmaceutic Ingredients, Patient related factors like age, Routes of administration (Parenteral, Rectal, Oral, and Topical) etc.
Conducting a BA study enables assessment of the impact of route of administration on BA and defines the absolute BA of the drug released from the drug product. BA for a given formulation provides an assessment of the relative fraction of the orally administered dose that is absorbed into the systemic circulation. If the reference standard is an IV dose, it is referred as Absolute Bioavailability. If the reference standard is any other dosage form than IV it is referred as Relative Bioavailability.
Track 4: Bioavailability Studies and Assessment
Conducting a Bioavailability study enables assessment of the impact of route of administration on BA and defines the absolute bioavailability of the drug released from the drug product. BA for a given formulation provides an assessment of the relative fraction of the orally administered dose that is absorbed into the systemic circulation. If the reference standard is an IV dose, it is referred as Absolute Bioavailability. If the reference standard is any other dosage form than IV, it is referred as Relative Bioavailability. Micro and Micro nutrients play a vital role in bioavailability
Bioavailability is a subcategory of pharmacological absorption. Bioavailability is generally assessed by finding the area under the plasma concentration–time curve. The factors affecting bioavailability are Pharmaceutics factors, physicochemical properties of drug, Dosage form characteristics & Pharmaceutical Ingredients, Patient related factors like age, Routes of administration (Parenteral, Rectal, Oral, and Topical) etc. Topics under this track includes Nutrient Bioavailability, Absolute Bioavailability, Relative Bioavailability, Mineral Bioavailability- Micro and Macro, Vitamins Bioavailability, BA of Contaminants in Soils & Sediments, Drug Absorption and Distribution, Disposition studies, Drug Formulation and Dosage Forms, Product design- Considerations, Bio accessibility Factor.
Track 5: Contract Research Organizations
A contract research organization (CRO) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to governmental organizations. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs that specialize in clinical-trials services can offer their clients the expertise of moving a new drug or device from its conception to FDA/EMA marketing approval, without the drug sponsor having to maintain a staff for these services.
Track 6: Recent approaches to Biosimilars
The development of biologics calls for overcoming lot many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors and labeling requirements. Also, it is much required now to develop a drug product in accordance to quality by design (QbD). This Drug related conference will look at the multiple facets of current challenges in bio similar development and focuses on multiple aspects of bio similar product development to successfully deliver safe, potential and efficacious biologic products to the market
Track 7: Pharmaceutical Freeze Drying Technology
Pharmaceutical freeze Technology is the removal of ice or other frozen solvents from a material through the process of sublimation and the removal of bound water molecules through the process of desorption. Lyophilization and freeze drying are terms that are used interchangeably depending on the industry and location where the drying is taking place. Controlled freeze drying keeps the product temperature low enough during the process to avoid changes in the dried product appearance and characteristics. It is an first-rate method for preserving a wide diversity of heat-sensitive materials such as proteins, microbes, pharmaceuticals, tissues & Plasma
Track 8: Recent advancements in BA/BE Research
The aim of bioavailability study is to find out the dosage form influence on the biological performance of the drug, sensitivity to detect differences in the rate and extent of absorption. Bioavailability and bioequivalence study design involves Single dose or multi dose standard 2x 2 crossovers, Parallel groups, for more than two formulations. Study design meant for estimating essential pharmacokinetic parameters differs significantly from a bioequivalence study meant for comparing the test formulation. The results of a pilot study can be used as the sole basis to document BA or BE provided the study’s design and execution are suitable and enough subjects have completed the study.
Track 9: Bioequivalence Studies and Assessment
Bioequivalence studies are done for Early and late clinical trial formulations, Formulations used in clinical trial and stability studies, if different Clinical trial formulations and to-be-marketed drug product when it comes to cost and productivity metrics, it’s often said that what gets measured gets done. Bioequivalence is determined based on the bioavailability of the innovator medicine versus the generic medicine. A typical outline for a bioequivalence study includes organization of the test and reference items on two events to volunteer subjects, with every organization isolated by a washout period. This Study involves parameters on (Cmax) and (AUC), Statistical evaluation.
Assessment of the bioequivalence of generic versions of certain reference drugs is complicated by the presence of endogenous levels of said compounds which cannot be distinguished from externally derived compound levels following drug administration. If unaccounted for, the presence of endogenous compound biases towards equivalence in bioequivalence studies of these drugs. Bioequivalence assessments may be complicated further as disposition of the exogenous analogue can be subject to various endogenous processes resulting in nonlinear pharmacokinetics. To overcome these inherent biases a number of different strategies have been employed.
Track 10: Nano Medicine & Nano Technology
Nanotechnology is the study of extremely small structures, having size of 0.1 to 100 nm. Nano medicine is a relatively new field of science and technology. Brief explanation of various types of pharmaceutical nano systems is given. Classification of nano materials based on their dimensions is given. An application of Nanotechnology in various fields such as health and medicine, electronics, energy and environment, is discussed in detail. Applications of nano particles in drug delivery, protein and peptide delivery, cancer are explained. Applications of various nano systems in cancer therapy such as carbon nano tube, dendrimers, nano crystal, nano wire, nano shells etc. are given. The advancement in nano technology helps in the treatment of neuro degenerative disorders such as Parkinson’s disease and Alzheimer’s disease. Applications of nano technology in tuberculosis treatment, the clinical application of nanotechnology in operative dentistry, in ophthalmology, in surgery, visualization, tissue engineering, antibiotic resistance, immune response are discussed in this article. Nano pharmaceuticals can be used to detect diseases at much earlier stages.
Track 11: Analytical Method Development and Validation
Analytical method development is the process of choosing an accurate assay procedure to determine the composition of a formulation. It is the process of proving that an analytical method is acceptable for use in laboratory to measure the concentration of subsequent samples Analytical methods should be recycled within GMP and GLP environments and must be developed using the protocols and acceptance conditions set out in the ICH guidelines. Analytical method development and validation stand the continuous and inter-dependent task associated with the research and development, quality control and quality assurance departments. Analytical procedures play a critical role in equivalence and risk assessment, management. It supports in formation of product-specific acceptance criteria and stability of results. Validation should establish that the analytical procedure is suitable for its intended purpose. Design of experiment is a powerful tool for the method characterization and validation.
Track 12: Advances in Chromatography and Mass Spectrometry
Chromatography and mass qualitative analysis is employed for analysis of organic compounds. Electro spray ionization (ESI) could be a method employed in mass spectroscopic analysis. As relate to chromatography and mass spectrometry. HPLC is further flexible informative and trusted by the industry people. Recent advances in sample preparation techniques to beat difficulties encountered throughout measuring of little molecules from bio fluids mistreatment LC-MS. For Measuring, observation and protective your important Investments analytical chemistry instruments are used. Global Bio analysis seminars are conducted and those specifically applied for chromatography assays, ligand binding assays to know more advances.
Track 13: Novel Approaches to Analytical and Bio analytical Methods
Analytical chemistry is that the study of the separation, identification, quality control and quality assurance of the chemical parts of natural and artificial materials .The maintenance of a desired level of quality in an exceptionally package or product, particularly by resources that of attention to every one stage of the method of delivery or production. Bio analytical Chemistry could be a sub-division of Analytical Chemistry that refuges the measuring of medicine, Ion sensors, Proteins and DNA Sequences in unnatural samples or concentrations. Accurate quantification of the drug samples is extremely very important for several scientific endeavors which cannot delay the result. Therefore the Bio analytical Techniques are in the main focused to bring the correct results of the drug sample to supply an ideal result.
Track 14: Latest Drug Development instruments
Our research instruments are designed to assist you in finding highly valuable and rare biological variants among vast cell populations. By increasing speed and reducing cost, our Pico droplet technology can help you save resources whilst boosting your chances of success. Our industrial instruments, our research instruments are semi-automated rather than fully automated. While this means they require slightly more user input to configure, it also means that they are more flexible and can be easily adapted to fit the needs of your unique research project.
Track 15: Role of Chemistry in Pharmaceuticals
Pharmaceutical science is a major field. It involves different areas of science, which are used to bring out pharmaceutical drug compounds. Computational chemistry involved with the design of new chemical compounds as drugs. Pharmaceutical chemistry and Organic chemistry are involved in the preparation of pharmaceutical drugs. Analytical chemistry involves the quality control and analysis of the pharmaceuticals. Pharmaceutics address the problems in making drug formulations like capsules, tablets, injections etc. The area of pharmacology deals with the study of the efficacy and toxicities of newly designed pharmaceutical compounds.
Track 16: Industrial Pharmacy& Physical Pharmacy
Industrial Pharmacy also plays a crucial role in any drug discovery. To any novel drug discovery the industrial approach is very important to get massive commercial application. Few things which have to be considered by industries to provide a safe and cost affective medicine to the patients like Supply chain, Waste management, Product management, Post- marketing surveillance, Good manufacturing practices and Marketing.
The U.S. pharmaceutical market is the world’s most important national market. Together with Canada and Mexico, it represents the largest continental Pharma market worldwide. The United States alone holds some 40 percent of the global pharmaceutical market. In 2014, this share was valued around 365 million U.S. dollars. Many of the global top companies are located in the United States. In 2014, six out of the top eleven companies were U.S.-based.
Track 17: NMR and Analysis of Small Organic Molecules
In bioscience and drugs, to a small grade molecule may be a coffee mass (<900 Daltons [1]) compound which is incapable to facilitate regulate a process, with a size on the order of 10−9 m. Most drug analysis square measure identical little molecules. For analysis of small organic molecules the subsequent devices ought to be recycled are as follows HPLC method, chromatography, Ultraviolet-visible (UV-VIS) spectrophotometry, Infrared (IR) spectrometry and mass spectrometry.
Track 18: Pharma Regulatory affairs
Regulatory affairs (RA), is also called government affairs, is a profession within regulated industries, such as pharmaceuticals, medical devices, energy, banking, telecom etc. Regulatory affairs also has a very specific meaning within the healthcare industries (pharmaceuticals, medical devices, biologics and functional foods)
Regulatory affairs (medical affairs) professionals (aka regulatory professionals) usually have responsibility for the following general areas: Ensuring that their companies comply with all of the regulations and laws pertaining to their business, Working with federal, state and local regulatory agencies and personnel on specific issues affecting their business. i.e. working with such agencies as the Food and Drug Administration or European Medicines Agency(pharmaceuticals and medical devices); The Department of Energy; or the Securities and Exchange Commission(banking), Advising their companies on the regulatory aspects and climate that would affect proposed activities. i.e. describing the "regulatory climate" around issues such as the promotion of prescription drugs and Sarbanes-Oxley compliance.
11th Annual Congress on Drug Formulation and Analytical Techniques conference presenting the global analytics of the relevancy and usage medicinal according to the annual expenditure. According to the regional survey of the year 2019, the worldwide pharmaceuticals showcase was worth $934.8 billion in 2018and will reach $1170 billion in 2021, developing at 5.8%, concurring to a later pharma showcase investigate the report. This can be a quickened pace compared to 5.2% for a span of 12 months before 2017 but is slower than the other two huge healthcare fragments, therapeutic gear and healthcare administrations. The biggest pharma showcase all the worldwide pharmaceutical explanatory testing advertises estimate was esteemed at USD 4.4 billion in 2018 and it is evaluated to develop with CAGR of 8.1% over the estimated period. Expanding R&D speculations, expanding center on the item quality & security, control is imperative drivers of the development of the advertising. Expanding R&D speculations is one of the basic maintainability techniques. Within the later a long time, the R&D cost is expanding and expected to proceed to extend over the figure period. The pharmaceuticals industry is ruled by the U.S., which holds around 45% of the worldwide showcase share since it is driven by the administrative situation and the nearness of the well-established outsourcing framework. Driving industry players designate around 20% of their turnover to R&D to preserve a competitive edge. Increase in complexity and number of benchmarks, which a single item may comply with, is driving significant development within the pharmaceutical expository testing administrations outsourcers inclusive is for musculoskeletal drugs. These are medications for infections such as rheumatoid- and osteo- joint pain, osteoporosis, carpal burrow disorder, tendonitis, rotator sleeve tear, strong dystrophy, myasthenia gravis, lupus erythematosus, and others. Major drugs in this section incorporate Piroxicam Glaxo, Dolonex, Felden, and Piroxicam Pfizer. The fragment accounted for 14% of the worldwide add up to in 2018. Cardiovascular, oncology and anti-infective drugs are the moments third and fourth biggest markets.
Current and progressing changes in political, financial, social, mechanical, lawful and natural variables are affecting development within healthcare advertising, where drugs play an imperative portion. The following variables are all boosting healthcare showcase growth:
-
Reduced charges and lowered sedate costs within the USA GDP development of over 6% in China and India
-
Widespread populace maturing and inactive ways of life driving to expanded unremitting malady prevalence Industrialized information administrations in R&D empowering the utilize of clinical trial information in trial simulations
-
Lowered administrative boundaries for unused drugs within the USA High urban contamination levels expanding the rate of conditions like asthma
The components that influence the pharmaceutical market estimate to incorporate illness predominance, medicate reasonableness, customer states of mind, governance arrangements and a few supply-side components
Infection predominance is related to populace measure, age, hereditary legacy and behavior (irresistible illness frequency is lower where sanitation hones are superior; stationary ways of life moreover energize constant disease). Affordability is related to salary but moreover to medicate prices. Consumer states of mind incorporate eagerness to utilize elective treatments or doubt of taking drugs. Government arrangements influence repayment and who the payer is. Other government approaches decide the direction, which can be a noteworthy boundary to the dispatch of modern treatments. A major supply-side calculate is the accessibility of a suitable treatment, which may be a matter of amount, as in a plague, or of sedate disclosure and advancement.
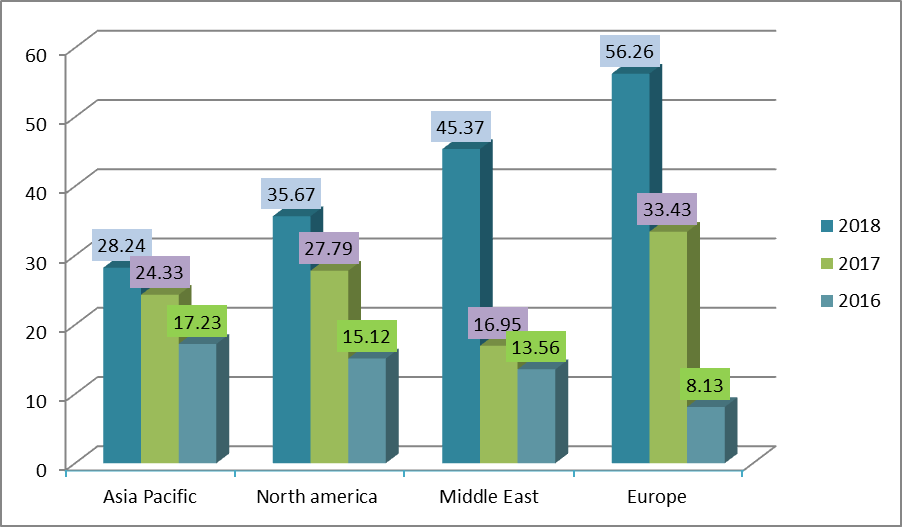
Global Pharmaceutical Market in the past 3 years
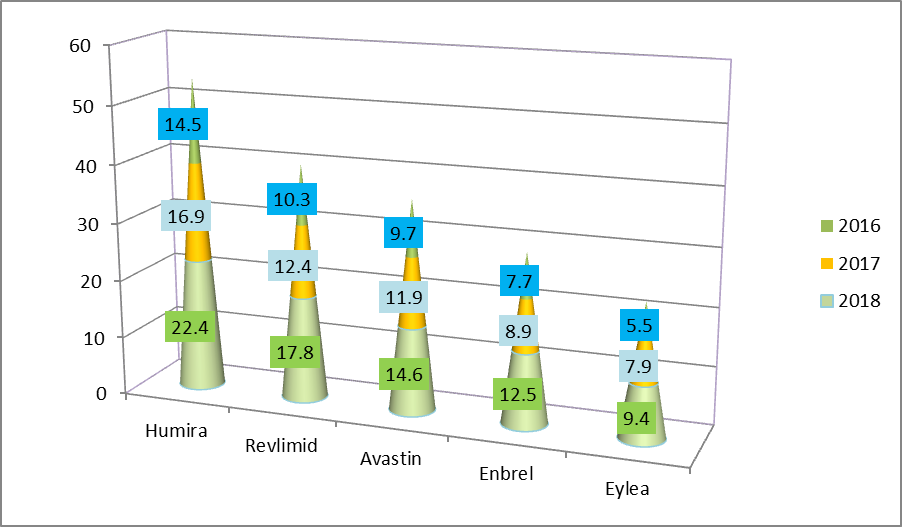
Sale Analysis of top drugs from last 3 years
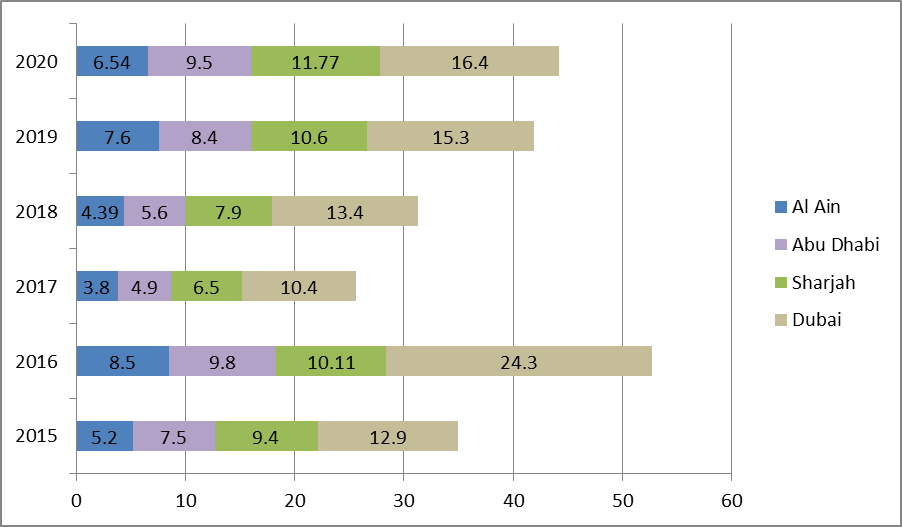
United Arab Emirates: pharmaceutical growth from 2015 to 2020
The measurement appears the (UAE) net pharmaceutical industrial development from 2012 to 2017, with projections up until 2020. In 2017, the United Arab Emirates pharma development rate produced to around 0.8 percent compared to the past year.
Treatment of Diabetes with Herbal Supplements
There are two primary sorts of diabetes: sort 1 and sort 2. Both sorts of diabetes are incessant illnesses that influence the way your body directs blood sugar or glucose. Glucose is the fuel that nourishes your body’s cells, but to enter your cells it needs a key. Insulin is that key. People with sort 1 diabetes don’t create insulin. You'll be able to think of it as not having a key. People with sort 2 diabetes don’t react to insulin as well as they ought to and afterward within the malady regularly don’t make sufficient insulin. You'll think of this as having a broken key. Both sorts of diabetes can lead to chronically tall blood sugar levels this causes increments and the chance of diabetes complications.
Causes and Symptoms of Diabetes:
The body’s immune system is dependable for battling off outside intruders, like destructive infections and microscopic organisms. In individuals with sort 1 diabetes, the resistant system mistakes the body’s claim solid cells for outside intruders. The safe framework assaults and devastates the insulin-producing beta cells within the pancreas. After these beta cells are crushed, the body is incapable to create insulin. Individuals with sort 2 diabetes have insulin resistance. The body still produces insulin, but it’s incapable to utilize it successfully. Analysts aren’t beyond any doubt why a few individuals gotten to be insulin resistance and others don’t, but a few lifestyle variables may contribute, counting overabundance weight and inactivity. Other hereditary and natural variables may moreover contribute. After you create sort 2 diabetes your pancreas will attempt to compensate by creating more insulin. Since your body is incapable to successfully utilize insulin, glucose will collect in your circulatory system.
Herbal Supplemental Treatment of Diabetes:
There are several herbs that involve in the treatment of diabetes or they involve in the regular controlling of an excess release of insulin or decreased secretion of insulin which leads to type 1 and type 2 diabetes. People suffering from type-2 diabetes can be treated with the following herbal supplements like
Aloe vera may be a common plant with numerous distinctive employments. Most individuals are mindful of the plant being utilized to coat the skin and ensure it from harm caused by as well much sun exposure. However, the plant has numerous lesser-known benefits as well. These extend from making a difference stomach related issues to conceivably indeed diminishing sort 2 diabetes side effects.
Cinnamon may be a fragrant herb made from the bark of a tree and is commonly found in kitchens. It incorporates a sweet and hot scent and taste that can include sweetness without any extra sugar. It is well known with individuals with sort 2 diabetes for this reason alone, but there's much more to cinnamon than fair flavors.
Momordica charantia moreover known as bitter melon could be a restorative fruit. It has been utilized for centuries within the conventional pharmaceutical of China and India. The severe natural product itself is cooked into numerous dishes, and the plant's restorative properties are still being found. Bitter melon seeds were given to both individuals with sort 1 and sort 2 diabetes to diminish their blood sugar levels. Mixed vegetable mash blended with water moreover brought down blood sugar levels in 86% of the sort 2 diabetes patients tried. The natural product juice of the bitter melon too made a difference to moved forward blood sugar resistance in numerous cases
Milk thistle is an herb that has been utilized since old times for numerous diverse afflictions and is considered a tonic for the liver. The foremost considered extricate from drain thorn is called silymarin, which could be a compound that has antioxidant and anti-inflammatory properties. It is these properties which will make milk thistle an extraordinary herb for individuals with diabetes.
Ginger is another herb that science is fair finding more almost. It has been utilized for thousands of a long time in conventional medication systems. Ginger is frequently utilized to assist treat stomach related and provocative issues. In any case, a later audit posted to appears that it may be accommodating in treating diabetes indications as well. Ginger is frequently included to nourishment crude or as a powdered herb, brewed into tea, or included to capsules as an herbal supplement.
Conference Highlights
- Pharmaceutical Formulations
- Formulation R&D
- Clinical Research and Clinical Trails
- Bioavailability Studies and Assessment
- Contract Research Organizations
- Recent approaches to Biosimilars
- Pharmaceutical Freeze Drying Technology
- Recent advancements in BA/BE Research
- Nano Medicine & Nano Technology
- Analytical Method Development and Validation
- Advances in Chromatography and Mass Spectrometry
- Novel Approaches to Analytical and Bio analytical Methods
- Latest Drug Development instruments
- Role of Chemistry in Pharmaceuticals
- Industrial Pharmacy& Physical Pharmacy
- NMR and Analysis of Small Organic Molecules
- Pharma Regulatory affairs
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | December 09-10, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by

